INVESTIGATOR: Doris Trauner, MD
STUDY LOCATION: University of California, San Diego
PROJECT TITLE: The Effects of Cannabidiol (CBD) on Symptoms of Severe Autism
FUNDING SOURCE: Wholistic Research and Education Foundation
PROJECT TYPE: Clinical Study
STATUS: COMPLETED
ABSTRACT:
This clinical trial is examing if and how cannabidiol (CBD), a chemical found in the cannabis plant, provides therapeutic benefit to children with severe autism spectrum disorder (ASD). The trial is funded by a grant from the Ray and Tye Noorda Foundation in partnership with and based on recommendations from the Wholistic Research and Education Foundation.
ASD affects an estimated one in 68 children in the United States, primarily boys. CBD is a major chemical compound found in cannabis. It does not produce the “highness” caused by THC but interacts with the body’s endocannabinoid system; a network that regulates diverse physiological and cognitive processes.
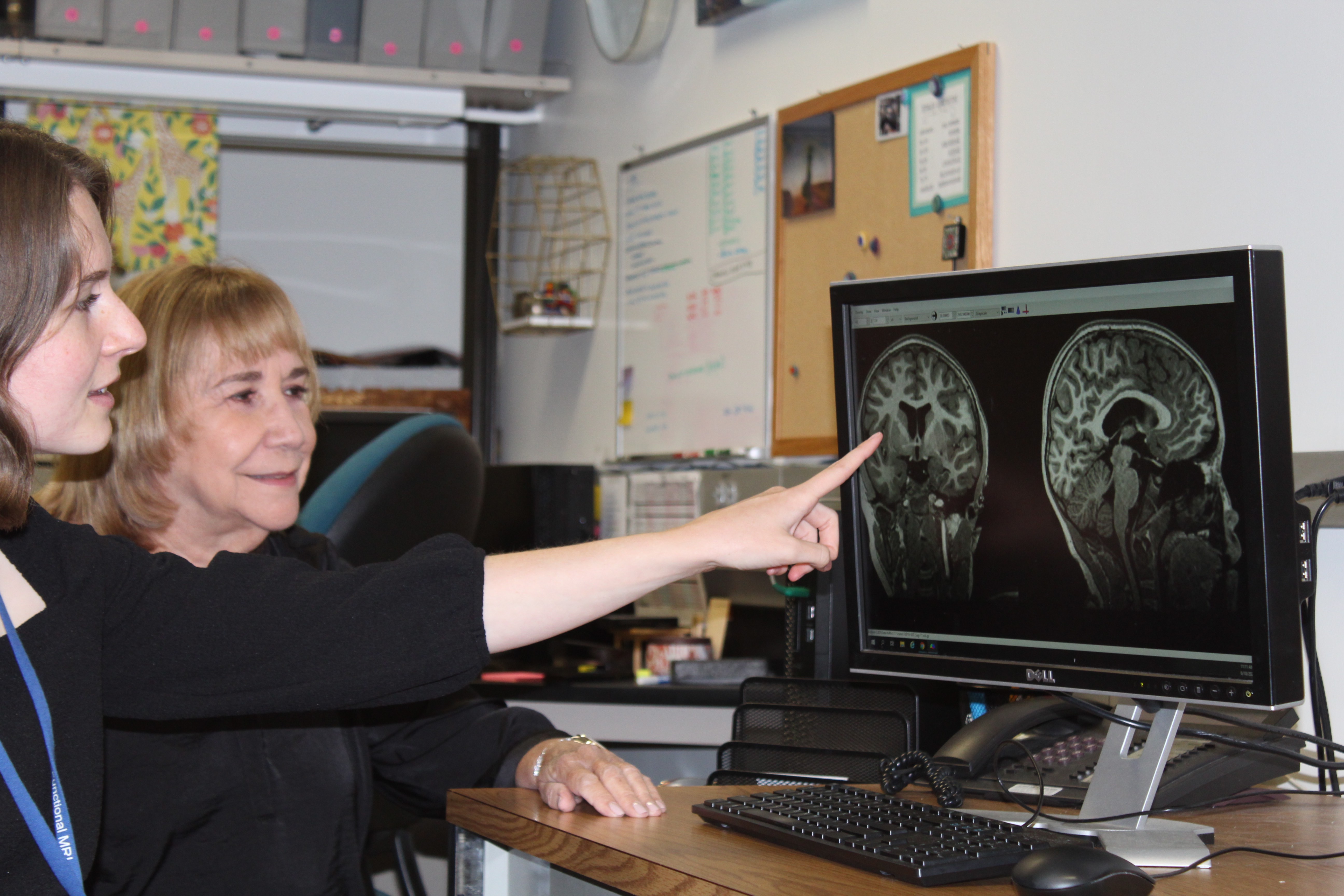
The goals of the study are to determine 1) if CBD is safe and tolerable and whether it helps with the symptoms of ASD; 2) whether and how CBD alters neurotransmitters and/or improves brain connectivity; and 3) whether biomarkers of neuro-inflammation, also associated with ASD, are altered by CBD.
The clinical trial consists of 30 children, ages eight to 12 years, with a confirmed diagnosis of moderate to severe autism. They must be free of other neurological conditions, such as epilepsy, and in general good health.
In the first phase of the study, half the children receive an oral dose of CBD and half placebo. In the second phase, the groups are switched and the half who originally received CBD receives placebo, while the initial placebo group receives CBD. Investigators are blinded to which children are receiving which treatment until after all of the testing is completed at the end of the study.